+86 17737144966
With the development of nuclear energy, radionuclide water pollution has become a major environmental problem. Often, water is radionuclide, either naturally or artificially, as a result of nuclear power plant accidents and leaks from spent fuel pools and nuclear fuel treatment facilities. Due to chemical and radiological toxicity, exposure to radionuclide contaminated water may present a more serious risk. Activated carbon-based adsorption technology has great potential in the treatment of radionuclide contaminated water due to its simple design, high efficiency, wide pH range, fast, low cost and environmental friendliness.
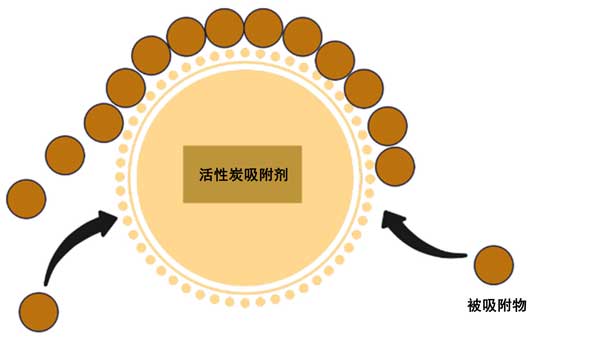
Adsorption technology is the transfer of molecules from the fluid body to the solid surface process called adsorption, as shown in Figure 1. Adsorption is a surface process that can be produced by chemical bonding or physical forces. This method can be divided into two different parts. The term“Adsorbent” refers to the type of molecules adsorbed on t
he surface, while“Adsorbent” describes the surface on which the adsorption process takes place. Activated carbon adsorption is the most popular and cost-effective removal method, which has almost no disadvantages and is still widely used. The adsorption performance of radionuclide depends to a large extent on the surface, physics and chemical property of the adsorbent. Therefore, the following section studies the performance of different adsorbents and adsorption conditions.
In a broad sense, activated carbon is an amorphous carbonaceous material characterized by high porosity and large intergranular surface area. It is widely used as an adsorbent. There are different types of activated carbon, such as granular, powdered, impregnated, extruded, bead, etc. . The most common type is powdered activated carbon. Similar to other adsorbents, surface area, porosity, pore volume, adsorption capacity, chemical inertia and stability are the main determining characteristics of better performance of activated carbon.
Previously, most organic resources could be used as precursors for the production of activated carbon. Today, biomass precursors are used more frequently because of their low cost, availability and reproducibility. Understanding the relationship between the porous properties of activated carbon, including surface area, pore size distribution, and adsorption capacity, is critical to selecting the most suitable adsorbent for effective radionuclide removal. Highly developed micropore and mesoporous structures are the decisive characteristics of porous carbon. For adsorption of smaller molecules, micropores are necessary. There are some pores smaller than 0.7 nm in diameter in activated carbon matrix. These pores are called ultramicropores. Micropores are the most important because of their large surface area and because of their small size, they have greater adsorption capacity. This is equivalent to molecules adsorbed in micropores. Molecules of a size between micropores and macropores are usually retained in the mesopore. A large hole is one that is too large to be filled by capillary condensation. The main purpose of macropores is to accelerate the transport of adsorbents to the pores located deeper inside the activated carbon. However, macropores may also contain macromolecules, such as humic acids, formed during the decomposition of organic compounds. Fig. 2 shows the hierarchical porous structure of activated carbon.
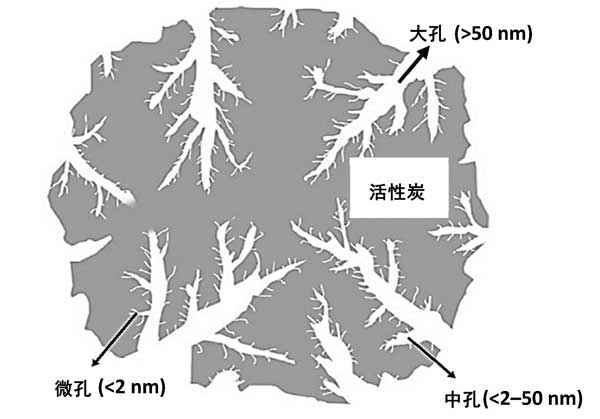
Fig. 2: the porous structure of activated carbon.
Activated carbon is a widely used effective adsorbent, because of its extremely high porosity, developed internal microporous structure and the existence of surface functional groups, it can be used to remove various pollutants dissolved in water medium or gas environment. Recent studies have shown that modified activated carbon surfaces can improve adsorption capacity. Because we are not sure which nuclides are adsorbed, we can not change what is adsorbed. However, adsorbents (in this case activated carbon) can be modified; therefore, their adsorption capacity is increased. These are physical modifications, chemical modifications and organic or inorganic load changes. In the case of physical modification, the surface of activated carbon is modified by microwave heating, ultraviolet radiation, steam modification and other physical activation techniques. Modern researchers are studying different methods to develop surface properties of activated carbon by varying pyrolysis temperature, heating time and heating rate, which may include ultraviolet and microwave radiation during pyrolysis. Many activated carbons have been modified and functionalized to improve radionuclide adsorption. The researchers used strong acids, alkalis, polymers and other synthetic materials to alter the functional groups and surface areas of activated carbon. Therefore, the specific surface area and adsorption capacity of activated carbon are enhanced. Various organic and inorganic substances can also be used to study the connection of functional oxygen groups to carbon.
Different radionuclide require different types of activated carbon to be functionalized to increase adsorption capacity or removal properties. Sulfonic acid was added to CO2-derived porous carbon composites to increase strontium removal. Radioactive cesium has been studied. By modifying the surface of activated carbon with covalent organic polymer, the impregnating adsorbent of Prussian blue was developed. The initial cesium removal rate of powdered activated carbon was 20% . However, the removal rate of cesium was as high as 86.7% after surface modification. Activated carbon derived from biomass precursors was modified with nitric acid and acetic acid. The iodine removal capacity of porous activated carbon was 806 mg/g. However, the adsorption capacity increased to 1385.5 mg/G after modified with nitric acid and to 1284.5 mg/G after modified with acetic acid. Activated carbon was impregnated with 2% WT/NaOH in another study to remove iodine 131. This increases the removal efficiency from 54% to 99.65% . Figure 3 shows a comparison of radionuclide removal efficiencies before and after modification of the parent material. It can be seen that the removal efficiency after modification is significantly improved
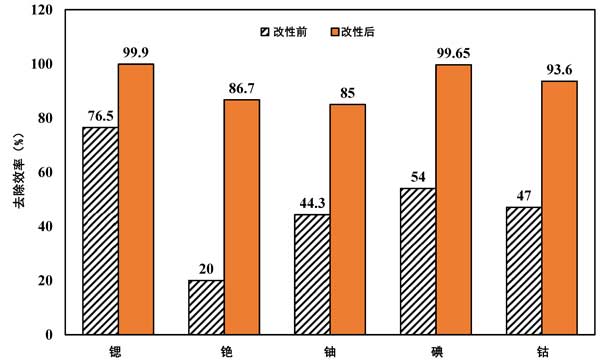
Figure 3: Comparison of radionuclide removal efficiency before and after activated carbon modification.
JS Activated carbon adsorption for removal of radionuclide from wastewater shows that adsorption technology using a variety of adsorbent materials has greater potential than other technologies. Activated carbon is regarded as a high quality material because of its special structure, rich functional group and possibility of additional functionalization. More importantly, it can be produced from abundant waste biomass resources and is cost-effective and renewable. It has been observed that the properties of biomass precursors, activators, activation conditions and surface properties are the key factors affecting the adsorption capacity of activated carbon. In addition, this review focuses on the modification of activated carbon properties to improve radionuclide adsorption. It was found that the removal efficiency of modified activated carbon was significantly improved, and the development of modified activated carbon for better removal may be an emerging research area. Depending on its performance, adsorption capacity and cost, it appears to be more feasible than other radionuclide removal technologies. Although substantial progress has been made in the development of activated carbon and its removal of radionuclide from wastewater, however, there is still an urgent need for further studies to find the optimal synthesis conditions, the optimal adsorption conditions and suitable reagents for modifying specific radionuclide.