+86 17737144966
activated carbon">Activated Carbon materials are widely used in industry and water treatment technology. The most popular applications, however, are in the removal of heavy metals. There are many other substances present in wastewater. Therefore, the simultaneous removal of molecules with various chemical properties is a very important issue that needs to be explained and extensively studied. Adsorption on the surface of activated carbon adsorbents can also be successfully used for the remediation of all types of pollutants in water. Due to their wide application in various branches of industry, agriculture and ecology, polymers are abundant in wastewater. They are usually synthetic substances whose effects on living organisms have not been fully explained and may be potentially dangerous. For this reason, a good method of removing polymers from the aquatic environment is required, and one of the technologies that meets this standard is the use of activated carbon adsorption.
The adsorbent used was activated carbon made from two precursors, both impregnated with 50% phosphoric acid and heated in a nitrogen atmosphere, with the first step at 200 ° C (5 ° C/min) for 30 minutes, the second is at 500 ° C (5 ° C/min) for 30 minutes. The obtained activated carbon is expressed as activated carbon 1 and activated carbon 2, respectively. The structure characterization of activated carbon is based on the nitrogen adsorption isotherm measured on an adsorbent ASAP at -196 ° C. Polyacrylic acid and Polyethylenimine are used as adsorbents. They are characterized by a weight-average molecular weight of 2000Da. The Polyacrylic acid is a weak polyelectrolyte with an anionic property (the carboxyl group is present in its macromolecules) and a PKA value of about 4.5. Thus, ionization is minimal at a pH of 3 for all measurements. The Polyethylenimine is a cationic polymer in which the amino group is present and has a PKB value of about 9. At a pH of 3, Polyethylenimine molecules appear in complete dissociation.
Adsorption characteristics of activated carbon in single and mixed solutions of Polyacrylic acid and Polyethylenimine at different temperatures, the amount of adsorption (expressed as Mg/g and polymer removal percentage) of both polymers on the surface of activated carbon in single and mixed adsorbent systems at ph 3 is shown in figures 1 and 2.
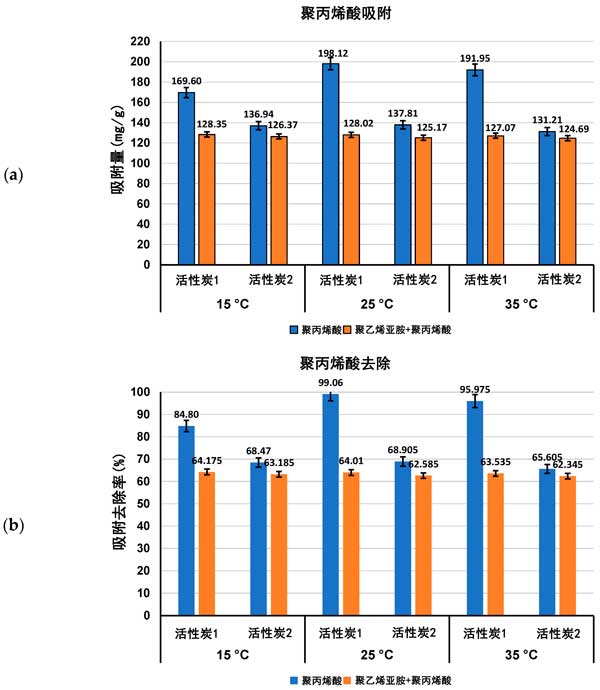
Figure 1: Polyacrylic acid adsorption (a) and its percentage removed from the surface of activated carbon (B) in single and mixed adsorbent systems at three detection temperatures pH 3.
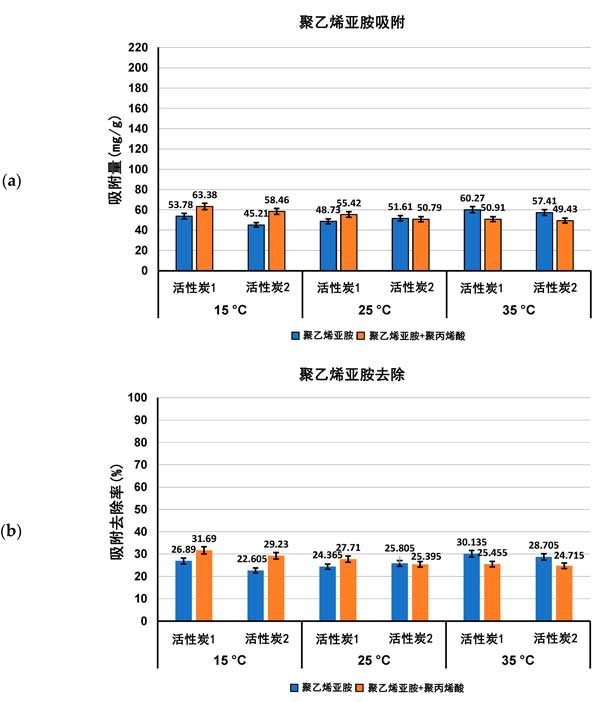
Figure 2: Polyethylenimine adsorption (a) and its percentage removed from the surface of activated carbon (B) in single and mixed adsorbent systems at three detection temperatures ph 3.
Previous studies of activated carbon surface Charge density have confirmed the acidity of both adsorbents. The zero-charge point (pzc) of activated carbon 1 occurs at ph 3.1 and, if activated carbon 2, at ph 4.0. This means that at a pH value of 3 for polymer adsorption, the surface of activated carbon 1 is almost neutral, while the surface of activated carbon 2 is slightly positively charged. As a result, electrostatic conditions have little effect on polymer adsorption at pH 3, and only weakly electrostatic attraction occurs on the surface of activated carbon 2 for the least dissociated Polyacrylic acid macromolecules. As the content of functional groups is quite high and the average pore size is significantly larger in the case of activated carbon 1, the adsorption of Polyacrylic acid on the surface of this carbonaceous material is higher than that on the surface of activated carbon 1 at all temperatures examined, either from a single adsorbent system or a mixed adsorbent system. Due to the complete dissociation of its functional groups and more tensile conformations leading to the blockage of the solid pores, Polyethylenimine adsorption is quite low.
For Polyacrylic acid (Fig. 1) , the effect of temperature on the adsorption capacity of the tested polymers was significantly greater than that of Polyethylenimine (Fig. 2) . The largest difference occurred on activated carbon 2, which adsorbed less at 15 ° C than at 25 ° C and 35 ° C, and it was noted that adsorption reached a maximum at 25 ° C (about 198 mg/g) . At 15 â ° C, the Polyacrylic acid macromolecule has an almost ideal conformation (with a fluid dynamics radius of at least 0.53 nm) . This coiling conformation limits the exposure of polymer functional groups to solid surfaces (which interact primarily to form hydrogen bonds) and minimal levels of Polyacrylic acid adsorption are observed. Furthermore, near Theta and critical point conditions reduce the solubility of polymer coils in water, which affects their tendency to accumulate at the interface. At 25 â ° C, the maximum adsorption of Polyacrylic acid on the surface of activated carbon 1 may be due to the small accumulation of polymer segments in the adsorption coil, the larger size (RH assumed 0.77 nm) and the higher affinity of the polymer to the solvent molecule improve its quality. These factors enable the carboxyl group of the polymer to effectively interact with the surface of the activated carbon 1 adsorbent and allow the macromolecule to effectively penetrate into the solid pore. On the other hand, at 35 â ° C, the Polyacrylic acid adsorption is about 6 mgg less than at 25 â ° C, due to the further increase of the fluid dynamics radius of the polymer chain, resulting in a flatter Polyacrylic acid conformation, the chains on the surface of the positively charged activated carbon 1. This results in a partial blockage of the inlet of the solid pore for adsorbing macromolecules. In the case of Polyethylenimine and mixed adsorbent systems, the temperature effect is quite small, and in most cases it remains within the measurement error range.
XPS study on the polymer adsorption layer formed on the surface of activated carbon at 25 ° C, xPS spectra of activated carbon samples before and after Polyacrylic acid and Polyethylenimine adsorption (figures 3 and 4) confirmed the formation of polymer film on the surface of the tested activated carbon. As a result of poly (acrylic acid) adsorption, a significant decrease in carbon content was observed along with an increase in oxygen contribution. This demonstrates that polymers (rich in carboxyl groups) are permanently bonded to the surface of the adsorbent. A slightly different picture was observed for systems containing activated carbon and Polyethylenimine. The contribution of both nitrogen and oxygen is increased by the adsorption of polymers containing nitrogen-rich functional species. However, the nitrogen content was almost three times that of systems without polymers. This clearly indicates the permanent binding of the polymer to the surface of the activated carbon. We have a very similar relationship in the case of systems containing both carbonaceous adsorbents and two polymers. The increase in the content of the two heteroatoms is even greater than that of the previously discussed system. This may indicate the interaction between individual polymer molecules.
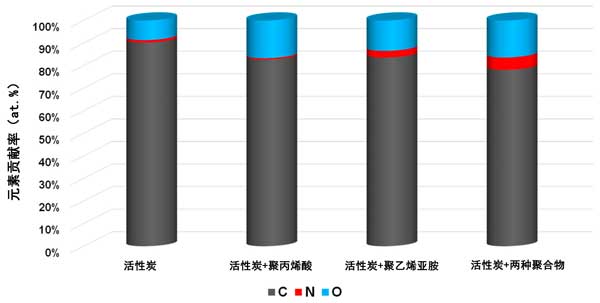
Figure 3: elemental composition of activated carbon surface before and after adsorption of polymer at 25 ° C.
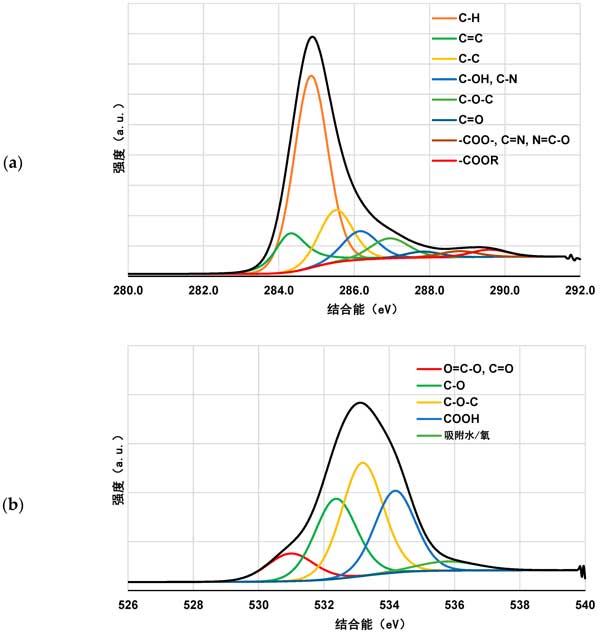
Figure 4: C 1s (a) and O 1s (B) spectra of activated carbon before polymer adsorption.
The effect of activated carbon on the removal of ionic polymers from aqueous solutions, activated carbon from chemically activated raw materials can be used as an effective adsorbent for Polyacrylic acid and Polyethylenimine in aqueous solutions at different temperatures (ranging from 15 ° C to 35 ° C) . As the temperature increases, the linear size of the polymer chains of both tested polymers (expressed as the root-mean-square (RMS) end-to-end distance and fluid dynamics radius) increases. Near the Polyacrylic acid coil at 15 â ° C (plus minimal dissociation at PH3) has a minimum size (fluid dynamics radius of 0.53 nm) . The linear size of the Polyethylenimine macromolecule is larger than that of the Polyacrylic acid macromolecule due to the complete dissociation of the polyethylene functional group at ph 3. The effect of temperature on the adsorption capacity of Polyacrylic acid polymers is larger than that of Polyethylenimine polymers. The largest difference occurred on activated carbon, which reached the maximum at 25 ° C (about 198 mg/g) . This may be due to the smaller accumulation of polymer segments in the adsorption coil and the increased affinity of the polymer for solvent molecules (facilitating macromolecules to permeate into solid pores) . Thermodynamic studies show that the adsorption process of polymers is spontaneous and endothermic, mainly through the formation of hydrogen bonds to achieve.