+86 17737144966
Wearing a mask in public is one of the most effective protective measures against the global spread of a pandemic virus. While masks are known to filter out viruses, dust, and harmful gases, masks with a layer of activated carbon">activated carbon may improve filtration efficiency. While these masks are usually adequate, they may have limitations in protecting against certain gases, toxic vapors, and very small pathogens at a high respiratory rate. The antibacterial efficiency of activated carbon fiber is more than 90% . It is found that the electrostatic attraction between virus and carbon structure can lead to the adsorption of virus on activated carbon fiber. Generally, activated carbon has a high surface area to effectively remove viruses, bacteria, VOC, odors and other gaseous pollutants from the air. The active carbon with 20-50nm pores can adsorb virus efficiently, and the protective ability of the mask has been improved by several grades with the addition of the active carbon layer.
The pore structure and carbon percentage of activated carbon are directly related to the adsorption performance and its application. Therefore, the proper pore size of activated carbon must be selected for correct application. For example, if the activated carbon sample has a microporous structure (& LT; 2 NM) , it will not be able to effectively adsorb mesoporous size (2-50 nm) gas molecules, such as those of some viruses, at approximately 60-140 nm. In order to accurately determine the adsorption capacity of activated carbon, it is necessary to study its isotherm, surface area and pore size distribution. Some commercial masks have been produced with bags for replaceable filters that contain activated carbon layer inserts. Although some studies have been carried out on the filtration efficiency of masks and the effect of pore morphology, size and distribution on the adsorption material, however, the research on the surface characteristics of the replaceable filter for virus removal is limited. Therefore, this study aims to determine whether commercial masks with activated carbon are better than conventional masks by focusing on the physical adsorption efficiency of activated carbon layers purchased from multiple manufacturers in commercially replaceable filters.
Analysis of the surface area of an adsorbent is essential because its adsorption capacity depends to a large extent on it. In order to obtain critical information about the adsorption process, N2 Sorption isotherm are usually used to determine the surface area, pore volume, and pore size distribution of the adsorbent. It is commonly used for surface analysis. Analysis of the pore size distribution of activated carbon is necessary, because the difference in pore size can affect the adsorption capacity. To do this, N2 used instruments to analyze the surface area of Sorption isotherm, Brunauer, Emmett and Teller (Bet) , and the pore size distribution of the adsorbents. Prior to N 2 analysis, the dry mass of the sample was calculated by subtracting the weight of the empty sample pool from the weight of the sample pool. Each sample was regenerated at 120 ° C for more than 12 hours and then subjected to an in situ degassing process at the same temperature for 1 hour, successfully removing the adsorbed contaminants. Subsequently, the pore size distribution for each sample was estimated using Density functional theory (DFT) and analyzed three times (N = 3) for each sample. In this study, four kinds of filter elements with activated carbon mask were tested (Figure 1) . As a result, Filter 1 exhibits a completely different appearance than the other three.

Figure 1: four types of mask liners with an activated carbon layer were used for this study. Each image represents a different filter. Sorption isotherm
The Sorption isotherm of activated carbon has been studied using nitrogen (N2) adsorption analysis, a standard method. Figure 2 shows the N 2 Sorption isotherm of activated carbon at 77K, which is found to be a type I isotherm. The rapid rise of N 2 adsorption in the low relative pressure range confirmed the presence of microspores in all samples, as shown in Fig. 2. Obviously, activated carbon 4 had the lowest N 2 adsorption, while activated carbon 1 and activated carbon 2 had overlapping Sorption isotherm, and activated carbon 3 had the highest N 2 adsorption. This variability in data may be attributable to the different precursor materials used. In addition, except activated carbon 3, most of the pore volume of activated carbon is filled below p/Po ≈0.1. For activated carbon 3, the pore volume filling was less than p/Po ≈0.3. After the initial increase to 0.1, the isotherm gradually bends, indicating that the increment of further adsorption is small. With the increase of relative pressure, the adsorption of all samples was almost horizontal, indicating that saturation had been reached.
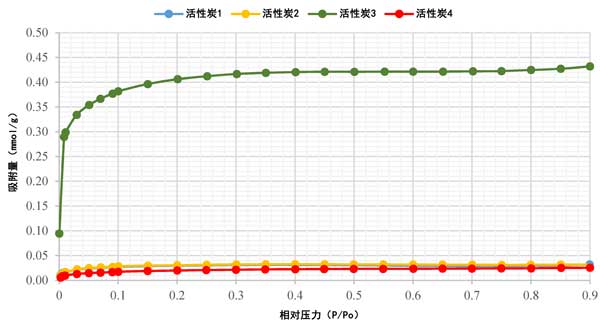
Figure 2: n 2 Sorption isotherm of activated carbon. Scanning electron microscope analysis (SEM)
At 1000 times magnification, individual fibers are clearly visible, and their cylindrical and smooth surfaces can be observed (fig. 3) . No porosity of the samples was observed in this study, which may be due to the small surface area of the samples, which makes them difficult to detect. In addition, activated carbon is not made entirely of carbon. As shown, the carbon layer is covered or sprayed with other materials. This may further lead to low surface area and therefore lack of porosity observations.
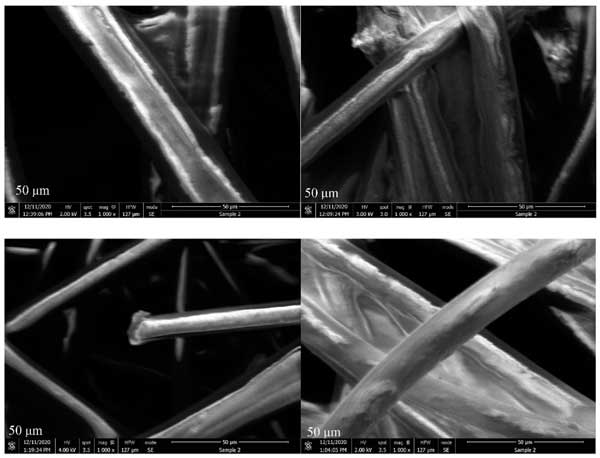
Figure 3: SEM image of activated carbon layer magnified 1000 times.
Activated carbon surface capture adsorption molecules, higher fixed carbon content indicates a larger carbon surface area. Therefore, the fixed carbon content is the main factor in the rough analysis. Typical activated carbon needs to have low ash content (2 -10%) , low moisture content (5 -8%) and high carbon content (85 -90%) . Although there are some criteria for the volatile content of powdered (25%) and granular AC(15%) forms of activated carbon, no criteria are available in the literature. However, because ash and volatile matter will fill the pores of activated carbon, so high volatile content is not ideal, resulting in a reduction of surface area, which has a negative impact on the adsorption performance of activated carbon. Activated carbon layer in the mask of physical adsorption performance, all samples have similar physical adsorption properties, compared with other samples, activated carbon 3 BET surface area slightly better, compared with other samples, carbon content is higher. However, despite their physical properties, all samples showed poor adsorption properties. Activated carbon 3 has two layers of activated carbon, but is not necessarily effective because of its small surface area. In order to filter virus effectively, activated carbon with high surface area (& GT; 1000) , high carbon percentage (& GT; 80%) and 0.1 micron pore size is recommended. The surface morphology of nonwoven fabric of all samples has been observed. This study only focused on the activated carbon in the mask filter, and concluded that the filter with activated carbon in the adsorption performance is not better than the filter without activated carbon. In addition, it has been revealed that the insert is not a pure carbonaceous material and that the polymer component may dominate.